MONSTAR-SCREEN

“To deliver the most promising drugs the fastest in the world “
Takayuki YoshinoPrincipal investigator of MONSTAR-SCREEN
Head, Department of Gastrointestinal Oncology, National Cancer Center Hospital East
What is MONSTAR-SCREEN?
- Industry-Academia collaborative project to investigate cancer genome alterations across a wide range of solid tumors.
- Introduction of blood-based circulating tumor DNA analysis technology (so-called liquid biopsy), which can reduce the burden on cancer patients.
- Longitudinal gut microbiome analysis potentially associated with cancer development and drug efficacy.
Project Aim
MONSTAR-SCREEN is an industry-academia collaborative cancer genome screening project for patients with a wide range of solid tumors.
The aim of this project is to offer the best treatment for each patient and to establish novel treatments by conducting not only tissue-based genome analysis but also blood-based one (liquid biopsy) as well as the gut microbiome analysis by feces.
- Registration period: June 2019~Mar 2021
- Planned number of subjects: 2,000 patients, twice on average (pre- & post- treatments)
- Target population: Advanced solid tumors(Gastrointestinal, Breast, Skin, Head and Neck, Gynecological, Urological tumors, etc.)
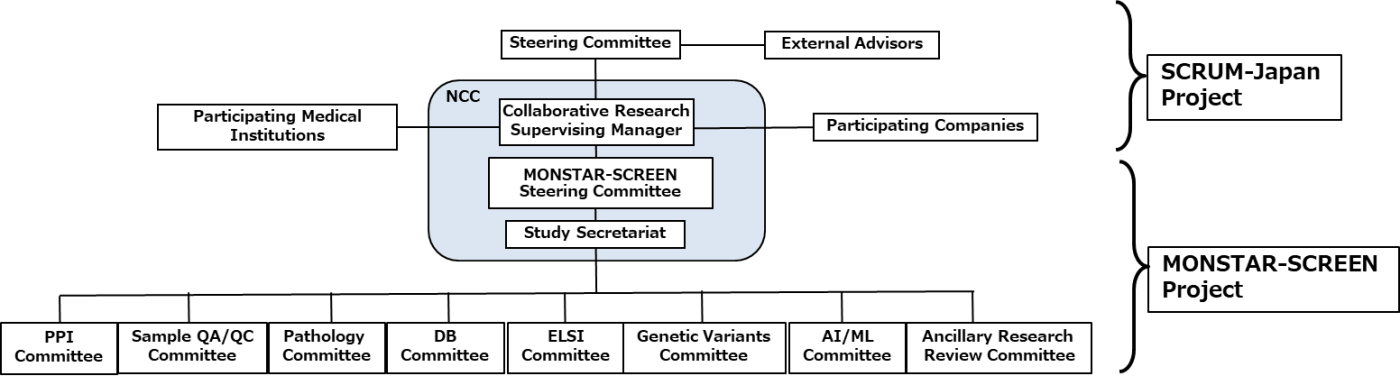
Structure of SCRUM-Japan&MONSTAR-SCREEN
Project Logo

Participating institutions for MONSTAR-SCREEN
- 1.Hokkaido University Hospital
- 2.University of Tsukuba Hospital
- 3.National Cancer Center Hospital East
- 4.Chiba Cancer Center
- 5.Saitama Cancer Center
- 6.National Cancer Center Hospital
- 7.Keio University Hospital
- 8.Kyorin University Hospital
- 9.Cancer Institute Hospital of JFCR
- 10.St.Marianna University School of Medicine Hospital
- 11.Kanagawa Cancer Center
- 12.Shizuoka Cancer Center
- 13.Kanazawa University Hospital
- 14.Aichi Cancer Center Hospital
- 15.Osaka University Hospital
- 16.Kindai University Hospital
- 17.Kansai Rosai Hospital
- 18.National Hospital Organization Shikoku Cancer Center
- 19.National Hospital Organization Kyushu Cancer Center
- 20.Kyushu University Hospital
- 21.National Hospital Organization Osaka National Hospital
- 22.Kagawa University Hospital
- 23.Saitama Medical University International Medical Center
- 24.Kobe City Medical Center General Hospital
- 25.Gifu University Hospital
- 26.Osaka Medical and Pharmaceutical University Hospital
- 27.Shimane Prefectural Central Hospital
- 28.Kansai Medical University Hospital
- 29.Kyoto Katsura Hospital
- 30.Osaka International Cancer Institute
- 31.Osaka General Medical Center
List of publications
- Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, Esaki T, Komatsu Y, Kawamoto Y, Takahashi N, Ueno M, Kagawa Y, Nishina T, Kato T, Yamamoto Y, Furuse J, Denda T, Kawakami H, Oki E, Nakajima T, Nishida N, Yamaguchi K, Yasui H, Goto M, Matsuhashi N, Ohtsubo K, Yamazaki K, Tsuji A, Okamoto W, Tsuchihara K, Yamanaka T, Miki I, Sakamoto Y, Ichiki H, Hata M, Yamashita R, Ohtsu A, Odegaard JI, Yoshino T. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020 Dec;26(12):1859-1864. doi: 10.1038/s41591-020-1063-5. Epub 2020 Oct 5. PMID: 33020649.
- Kotani D, Bando H, Taniguchi H, Masuishi T, Komatsu Y, Yamaguchi K, Nakajima T, Satoh T, Nishina T, Esaki T, Nomura S, Takahashi K, Iida S, Matsuda S, Motonaga S, Fuse N, Sato A, Fujii S, Ohtsu A, Ebi H, Yoshino T. BIG BANG study (EPOC1703): multicentre, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy with binimetinib, encorafenib and cetuximab in patients with BRAF non-V600E mutated metastatic colorectal cancer. ESMO Open. 2020;5(1):e000624. doi: 10.1136/esmoopen-2019-000624. Epub 2020 Sep 30. PMID: 33551068; PMCID: PMC7046405.
- Fujii S, Magliocco AM, Kim J, Okamoto W, Kim JE, Sawada K, Nakamura Y, Kopetz S, Park WY, Tsuchihara K, Kim TW, Raghav K, Yoshino T. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified Metastatic Colorectal Cancer Allowing for Screening by Next-Generation Sequencing Panel. JCO Precis Oncol. 2019 no. 4(2020)6-19. Published online January 7, 2020
- Hosono Y, Masuishi T, Yamaguchi R, Kato S, Yoshino T, Ebi H. Evaluation of ALK fusion newly identified in colon cancer by a comprehensive genomic analysis. JCO Precis Oncol. 2019 published online November 1, 2019
- Masuishi T, Taniguchi H, Kotani D, Bando H, Komatsu Y, Shinozaki E, Nakajima TE, Satoh T, Nishina T, Esaki T, Wakabayashi M, Nomura S, Takahashi K, Ono H, Hirano N, Fujishiro N, Fuse N, Sato A, Ohtsu A, Yoshino T. Rationale and design of the BRAVERY study (EPOC1701): a multicentre phase II study of eribulin in patients with BRAF V600E mutant metastatic colorectal cancer. ESMO Open. 2019 Nov 13;4(6):e000590. doi: 10.1136/esmoopen-2019-000590. PMID: 31798981; PMCID: PMC6863665.
- Yaeger R, Kotani D, Mondaca S, Parikh AR, Bando H, Van Seventer EE, Taniguchi H, Zhao H, Thant CN, de Stanchina E, Rosen N, Corcoran RB, Yoshino T, Yao Z, Ebi H. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin Cancer Res. 2019 Dec 1;25(23):7089-7097. doi: 10.1158/1078-0432.CCR-19-2004. Epub 2019 Sep 12. PMID: 31515458; PMCID: PMC6891165.
- Willis J, Lefterova MI, Artyomenko A, Kasi PM, Nakamura Y, Mody K, Catenacci DVT, Fakih M, Barbacioru C, Zhao J, Sikora M, Fairclough SR, Lee H, Kim KM, Kim ST, Kim J, Gavino D, Benavides M, Peled N, Nguyen T, Cusnir M, Eskander RN, Azzi G, Yoshino T, Banks KC, Raymond VM, Lanman RB, Chudova DI, Talasaz A, Kopetz S, Lee J, Odegaard JI. Validation of Microsatellite Instability Detection Using a Comprehensive Plasma-Based Genotyping Panel. Clin Cancer Res. 2019 Dec 1;25(23):7035-7045. doi: 10.1158/1078-0432.CCR-19-1324. Epub 2019 Aug 4. PMID: 31383735.
- Nakamura Y, Sawada K, Fujii S, Yoshino T. HER2-targeted therapy should be shifted towards an earlier line for patients with anti-EGFR-therapy naïve, HER2-amplified metastatic colorectal cancer. ESMO Open. 2019 Jul 8;4(3):e000530. doi: 10.1136/esmoopen-2019-000530. PMID: 31354963; PMCID: PMC6615873.
- Bando H, Kagawa Y, Kato T, Akagi K, Denda T, Nishina T, Komatsu Y, Oki E, Kudo T, Kumamoto H, Yamanaka T, Yoshino T. A multicentre, prospective study of plasma circulating tumour DNA test for detecting RAS mutation in patients with metastatic colorectal cancer. Br J Cancer 2019 ; 120 : 982-6.
- Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018 ; 29 : 44-70.
- Taniguchi H, Okamoto W, Muro K, Akagi K, Hara H, Nishina T, Kajiwara T, Denda T, Hironaka S, Kudo T, Satoh T, Yamanaka T, Abe Y, Fukushima Y, Yoshino T. Clinical Validation of Newly Developed Multiplex Kit Using Luminex xMAP Technology for Detecting Simultaneous RAS and BRAF Mutations in Colorectal Cancer: Results of the RASKET-B Study. Neoplasia 2018 ; 20 : 12 19-26.
- Nakamura Y, Yoshino T. Clinical Utility of Analyzing Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. Oncologist 2018 ; 23 : 13 10-8.
- Bando H, Okamoto W, Fukui T, Yamanaka T, Akagi K, Yoshino T. Utility of the quasi-monomorphic variation range in unresectable metastatic colorectal cancer patients. Cancer Sci 2018; 19 : 34 11-5.
Guidelines
- Ebi H, Bando H, Taniguchi H, Sunakawa Y, Okugawa Y, Hatanaka Y, Hosoda W, Kumamoto K, Nakatani K, Yamazaki K. Japanese Society of Medical Oncology Clinical Guidelines: Molecular Testing for Colorectal Cancer Treatment, 4th edition. Cancer Sci. 2020 Jul 15.
- Clinical Practice Guidelines for Tumor-Agnostic Treatments in Adult and Pediatric Patientswith Advanced Solid Tumors toward Precision Medicine.
Led by Japan Society of Clinical Oncology(JSCO) and Japanese Society of Medical Oncology(JSMO),cooperated by The Japanese Society of Pediatric Hematology/Oncology(JSPHO). Published October 24, 2019 - Mishima S, Taniguchi H, Akagi K, Baba E, Fujiwara Y, Hirasawa A, Ikeda M, Maeda O, Muro K, Nishihara H, Nishiyama H, Takano T, Tsuchihara K, Yatabe Y, Kodera Y, Yoshino T. Japan Society of Clinical Oncology provisional clinical opinion for the diagnosis and use of immunotherapy in patients with deficient DNA mismatch repair tumors, cooperated by Japanese Society of Medical Oncology, First Edition. Int J Clin Oncol 2019 [Epub ahead of print].
- Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Kato K, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Nakajima TE, Shitara K, Kawakami H, Tsushima T, Yoshino T, Lordick F, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY.Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019 ; 30 : 19-33.
- Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018 ; 29 : 44-70.
- Sunami K, Takahashi H, Tsuchihara K, Takeda M, Suzuki T, Naito Y, Sakai K, Dosaka-Akita H, Ishioka C, Kodera Y, Muto M, Wakai T, Yamazaki K, Yasui W, Bando H, Fujimoto Y, Fukuoka S, Harano K, Kawazoe A, Kimura G, Koganemaru S, Kogawa T, Kotani D, Kuboki Y, Matsumoto H, Matsumoto S, Mishima S, Nakamura Y, Sawada K, Shingaki S, Shitara K, Umemoto K, Umemura S, Yasuda K, Yoshino T, Yamamoto N, Nishio K, Japanese Society of Medical O, Japan Society of Clinical O, Japanese Cancer A. Clinical practice guidance for next-generation sequencing in cancer diagnosis and treatment (Edition 1.0). Cancer Sci 2018 ; 19 : 29 80-5.
Collaborating industries
- Amgen inc.
- CHUGAI PHARMACEUTICAL CO., LTD.
- Daiichi Sankyo Company, Limited
- Eisai Co., Ltd.
- Eli Lilly Japan K.K.
- Janssen Pharmaceutical K.K.
- Kyowa Kirin CO., LTD.
- MEDICAL & BIOLOGICAL LABORATORIES CO., LTD.
- MSD K.K.
- Nippon Boehringer Ingelheim Co ., Ltd.
- ONO PHARMACEUTICAL CO., LTD.
- Sumitomo Dainippon Pharma Co., Ltd.
- TAIHO Phamaceutical Co.,Ltd.
- Takeda Pharmaceutical Company Limited
